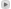 by Dreams End » Wed Jul 19, 2006 10:13 pm
by Dreams End » Wed Jul 19, 2006 10:13 pm
Not sure what the disconnect is.<br><br>Q.T. is a mathematical model. We can't "see" what happens at the atomic level. Though we can get really close with these types of electron scanning pics:<br><br><!--EZCODE IMAGE START--><img src="http://emusician.com/mag/504Tech-PageFig.-1.gif" style="border:0;"/><!--EZCODE IMAGE END--><br><!--EZCODE QUOTE START--><blockquote><strong><em>Quote:</em></strong><hr><br>FIG. 1: In this image from a �scanning �tunneling microscope, �electron-�density waves diffract around atom-sized pimples on the surface of a copper crystal, �demonstrating the wave nature of these particles.<hr></blockquote><!--EZCODE QUOTE END--><br><br>Lots of similar pics out there, showing quantum "fuzziness". <br><br>The issue of not being able to "find" electrons is not a limitation of technology but a property of the electrons. The "shapes" of the orbitals are, as posted above, areas where the electron is most likely to be found....the fact that it is a probability and not a precise location is the whole basis of quantum mechanics in the first place.<br><br>QT maybe overturned...happens all the time. However, the computer you typed on depends on all kinds of quantum weirdness to function. Your computer is based on semiconductors. They are built using the very sophisticated analysis available via Quantum Theory:<br><br><!--EZCODE QUOTE START--><blockquote><strong><em>Quote:</em></strong><hr>It is interesting to note that the reason semiconductors work is a direct result of quantum physics, in specific the Pauli exclusion principle. This principle states that no two particles can exist in the same state at the same time. Electrons and holes both behave in this way, and the entire electron-hole state is forced to follow certain energy distribution statistics, which basically mean that at any given temperature the distribution of free electrons and holes are <!--EZCODE QUOTE START--><blockquote><strong><em>Quote:</em></strong><hr>statistically de<hr></blockquote><!--EZCODE QUOTE END--><hr></blockquote><!--EZCODE QUOTE END--> and predictable. This also means that the conductivity of a semiconductor has a heavy temperature dependency, as a semiconductor operating at very low temperatures (-100°C or so) will have significantly fewer available free electrons and holes able to do the work. If you cool an IC down cold enough, the semiconductor will go intrinsic and all electrical signals will stop. (You would think that heating up the semiconductor has the opposite effect, but there are lots of other problems that happen at high temperatures, including loss of semiconducting properties due to too much free energy, so there is always a happy middle where the semiconductor wants to play!)[/quote]<br><br>They can't tell you WHICH electrons will go, but they can tell you statistically how MANY will go. QT says that WHICH one goes is truly random. You are in good company, though, as Einstein didn't like it much either...but a thought experiment he devised which was supposed to disprove it was turned into an actual experiment...which demonstrated that QT works (see EPR paradox).<br><br>Science uses QT because it is so far the most accurate predictor of observable behavior of atoms. Just like Newton's gravity...which did quite well except for little anomalies like a hitch in Mercury's orbit, another theory may come along which is even better at this...or maybe that can join quantum physics of the very small to gravitational physics of the very large (so-called Grand Unified Theorem).<br><br>But the point is that it works, and the little drawings are simply ways to help visualize what is not seeable (too tiny to bounce photons off of and get coherent results.) <br><br>Now, I will readily agree to the fact that carbon has six protons and six neutrons (Carbon 12, at least. All Carbon contains other isotopes of Carbon as well which means different numbers of neutrons.)<br><br>So I grant you that point...though for the life of me I'm not sure what the signficance of it is.<br><br><br><br> <p></p><i></i>
